Carbon
|
|||||||||||||||||||||||||
| Appearance | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
clear (diamond) & black (graphite) Spectral lines of Carbon |
|||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||
| Name, symbol, number | carbon, C, 6 | ||||||||||||||||||||||||
| Pronunciation | /ˈkɑrbən/ | ||||||||||||||||||||||||
| Element category | nonmetal | ||||||||||||||||||||||||
| Group, period, block | 14, 2, p | ||||||||||||||||||||||||
| Standard atomic weight | 12.0107(8)g·mol−1 | ||||||||||||||||||||||||
| Electron configuration | 1s2 2s2 2p2 or [He] 2s2 2p2 | ||||||||||||||||||||||||
| Electrons per shell | 2,4 (Image) | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| Phase | Solid | ||||||||||||||||||||||||
| Density (near r.t.) | amorphous:[1] 1.8–2.1 g·cm−3 | ||||||||||||||||||||||||
| Density (near r.t.) | graphite: 2.267 g·cm−3 | ||||||||||||||||||||||||
| Density (near r.t.) | diamond: 3.515 g·cm−3 | ||||||||||||||||||||||||
| Sublimation point | 3915 K, 3642 °C, 6588 °F | ||||||||||||||||||||||||
| Triple point | 4600 K (4327°C), 10800[2][3] kPa | ||||||||||||||||||||||||
| Heat of fusion | 117 (graphite) kJ·mol−1 | ||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 8.517(graphite), 6.155(diamond) J·mol−1·K−1 |
||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||
| Oxidation states | 4, 3 [4], 2, 1 [5], 0, -1, -2, -3, -4[6] | ||||||||||||||||||||||||
| Electronegativity | 2.55 (Pauling scale) | ||||||||||||||||||||||||
| Ionization energies (more) |
1st: 1086.5 kJ·mol−1 | ||||||||||||||||||||||||
| 2nd: 2352.6 kJ·mol−1 | |||||||||||||||||||||||||
| 3rd: 4620.5 kJ·mol−1 | |||||||||||||||||||||||||
| Covalent radius | 77(sp³), 73(sp²), 69(sp) pm | ||||||||||||||||||||||||
| Van der Waals radius | 170 pm | ||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[7] | ||||||||||||||||||||||||
| Thermal conductivity | (300 K) 119-165 (graphite) 900-2300 (diamond) W·m−1·K−1 |
||||||||||||||||||||||||
| Thermal expansion | (25 °C) 0.8 (diamond) [8] µm·m−1·K−1 | ||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 18350 (diamond) m/s | ||||||||||||||||||||||||
| Young's modulus | 1050 (diamond) [8] GPa | ||||||||||||||||||||||||
| Shear modulus | 478 (diamond) [8] GPa | ||||||||||||||||||||||||
| Bulk modulus | 442 (diamond) [8] GPa | ||||||||||||||||||||||||
| Poisson ratio | 0.1 (diamond) [8] | ||||||||||||||||||||||||
| Mohs hardness | 1-2 (Graphite) 10 (Diamond) |
||||||||||||||||||||||||
| CAS registry number | 7440-44-0 | ||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||
| Main article: Isotopes of carbon | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
Carbon (pronounced /ˈkɑrbən/) is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. There are three naturally occurring isotopes, with 12C and 13C being stable, while 14C is radioactive, decaying with a half-life of about 5730 years.[9] Carbon is one of the few elements known since antiquity.[10][11] The name "carbon" comes from Latin language carbo, coal.
There are several allotropes of carbon of which the best known are graphite, diamond, and amorphous carbon.[12] The physical properties of carbon vary widely with the allotropic form. For example, diamond is highly transparent, while graphite is opaque and black. Diamond is among the hardest materials known, while graphite is soft enough to form a streak on paper (hence its name, from the Greek word "to write"). Diamond has a very low electrical conductivity, while graphite is a very good conductor. Under normal conditions, diamond has the highest thermal conductivity of all known materials. All the allotropic forms are solids under normal conditions but graphite is the most thermodynamically stable.
All forms of carbon are highly stable, requiring high temperature to react even with oxygen. The most common oxidation state of carbon in inorganic compounds is +4, while +2 is found in carbon monoxide and other transition metal carbonyl complexes. The largest sources of inorganic carbon are limestones, dolomites and carbon dioxide, but significant quantities occur in organic deposits of coal, peat, oil and methane clathrates. Carbon forms more compounds than any other element, with almost ten million pure organic compounds described to date, which in turn are a tiny fraction of such compounds that are theoretically possible under standard conditions.[13]
Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. It is present in all known lifeforms, and in the human body carbon is the second most abundant element by mass (about 18.5%) after oxygen.[14] This abundance, together with the unique diversity of organic compounds and their unusual polymer-forming ability at the temperatures commonly encountered on Earth, make this element the chemical basis of all known life.
Contents |
Characteristics
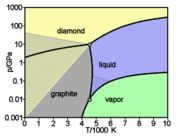
The different forms or allotropes of carbon (see below) include the hardest naturally occurring substance, diamond, and also one of the softest known substances, graphite. Moreover, it has an affinity for bonding with other small atoms, including other carbon atoms, and is capable of forming multiple stable covalent bonds with such atoms. As a result, carbon is known to form almost ten million different compounds; the large majority of all chemical compounds.[13] Carbon also has the highest melting and sublimation point of all elements. At atmospheric pressure it has no melting point as its triple point is at 10.8 ± 0.2 MPa and 4600 ± 300 K,[2][3] so it sublimates at about 3900 K.[15][16]
Carbon sublimes in a carbon arc which has a temperature of about 5800 K. Thus, irrespective of its allotropic form, carbon remains solid at higher temperatures than the highest melting point metals such as tungsten or rhenium. Although thermodynamically prone to oxidation, carbon resists oxidation more effectively than elements such as iron and copper that are weaker reducing agents at room temperature.
Carbon compounds form the basis of all known life on Earth, and the carbon-nitrogen cycle provides some of the energy produced by the Sun and other stars. Although it forms an extraordinary variety of compounds, most forms of carbon are comparatively unreactive under normal conditions. At standard temperature and pressure, it resists all but the strongest oxidizers. It does not react with sulfuric acid, hydrochloric acid, chlorine or any alkalis. At elevated temperatures carbon reacts with oxygen to form carbon oxides, and will reduce such metal oxides as iron oxide to the metal. This exothermic reaction is used in the iron and steel industry to control the carbon content of steel:
- Fe3O4 + 4 C(s) → 3 Fe(s) + 4 CO(g)
with sulfur to form carbon disulfide and with steam in the coal-gas reaction:
- C(s) + H2O(g) → CO(g) + H2(g).
Carbon combines with some metals at high temperatures to form metallic carbides, such as the iron carbide cementite in steel, and tungsten carbide, widely used as an abrasive and for making hard tips for cutting tools.
As of 2009, graphene appears to be the strongest material ever tested.[17] However, the process of separating it from graphite will require some technological development before it is economical enough to be used in industrial processes.[18]
The system of carbon allotropes spans a range of extremes:
| Synthetic nanocrystalline diamond is the hardest material known. | Graphite is one of the softest materials known. |
| Diamond is the ultimate abrasive. | Graphite is a very good lubricant. |
| Diamond is an excellent electrical insulator. | Graphite is a conductor of electricity. |
| Diamond is the best known naturally occurring thermal conductor | Some forms of graphite are used for thermal insulation (i.e. firebreaks and heat shields) |
| Diamond is highly transparent. | Graphite is opaque. |
| Diamond crystallizes in the cubic system. | Graphite crystallizes in the hexagonal system. |
| Amorphous carbon is completely isotropic. | Carbon nanotubes are among the most anisotropic materials ever produced. |
Allotropes
Atomic carbon is a very short-lived species and, therefore, carbon is stabilized in various multi-atomic structures with different molecular configurations called allotropes. The three relatively well-known allotropes of carbon are amorphous carbon, graphite, and diamond. Once considered exotic, fullerenes are nowadays commonly synthesized and used in research; they include buckyballs,[19][20] carbon nanotubes,[21] carbon nanobuds[22] and nanofibers.[23][24] Several other exotic allotropes have also been discovered, such as lonsdaleite,[25] glassy carbon,[26] carbon nanofoam[27] and linear acetylenic carbon.[28]
- The amorphous form is an assortment of carbon atoms in a non-crystalline, irregular, glassy state, which is essentially graphite but not held in a crystalline macrostructure. It is present as a powder, and is the main constituent of substances such as charcoal, lampblack (soot) and activated carbon.
- At normal pressures carbon takes the form of graphite, in which each atom is bonded trigonally to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The resulting network is 2-dimensional, and the resulting flat sheets are stacked and loosely bonded through weak van der Waals forces. This gives graphite its softness and its cleaving properties (the sheets slip easily past one another). Because of the delocalization of one of the outer electrons of each atom to form a π-cloud, graphite conducts electricity, but only in the plane of each covalently bonded sheet. This results in a lower bulk electrical conductivity for carbon than for most metals. The delocalization also accounts for the energetic stability of graphite over diamond at room temperature.
 Some allotropes of carbon: a) diamond; b) graphite; c) lonsdaleite; d–f) fullerenes (C60, C540, C70); g) amorphous carbon; h) carbon nanotube.
Some allotropes of carbon: a) diamond; b) graphite; c) lonsdaleite; d–f) fullerenes (C60, C540, C70); g) amorphous carbon; h) carbon nanotube. - At very high pressures carbon forms the more compact allotrope diamond, having nearly twice the density of graphite. Here, each atom is bonded tetrahedrally to four others, thus making a 3-dimensional network of puckered six-membered rings of atoms. Diamond has the same cubic structure as silicon and germanium and because of the strength of the carbon-carbon bonds, it is the hardest naturally occurring substance in terms of resistance to scratching. Contrary to the popular belief that "diamonds are forever", they are in fact thermodynamically unstable under normal conditions and transform into graphite.[12] But due to a high activation energy barrier, the transition into graphite is so extremely slow at room temperature as to be unnoticeable.
- Under some conditions, carbon crystallizes as lonsdaleite. This form has a hexagonal crystal lattice where all atoms are covalently bonded. Therefore, all properties of lonsdaleite are close to those of diamond.[25]
- Fullerenes have a graphite-like structure, but instead of purely hexagonal packing, they also contain pentagons (or even heptagons) of carbon atoms, which bend the sheet into spheres, ellipses or cylinders. The properties of fullerenes (split into buckyballs, buckytubes and nanobuds) have not yet been fully analyzed and represent an intense area of research in nanomaterials. The names "fullerene" and "buckyball" are given after Richard Buckminster Fuller, popularizer of geodesic domes, which resemble the structure of fullerenes. The buckyballs are fairly large molecules formed completely of carbon bonded trigonally, forming spheroids (the best-known and simplest is the soccerball-shaped structure C60 buckminsterfullerene).[19] Carbon nanotubes are structurally similar to buckyballs, except that each atom is bonded trigonally in a curved sheet that forms a hollow cylinder.[20][21] Nanobuds were first published in 2007 and are hybrid bucky tube/buckyball materials (buckyballs are covalently bonded to the outer wall of a nanotube) that combine the properties of both in a single structure.[22]
- Of the other discovered allotropes, carbon nanofoam is a ferromagnetic allotrope discovered in 1997. It consists of a low-density cluster-assembly of carbon atoms strung together in a loose three-dimensional web, in which the atoms are bonded trigonally in six- and seven-membered rings. It is among the lightest known solids, with a density of about 2 kg/m3.[29] Similarly, glassy carbon contains a high proportion of closed porosity.[26] But unlike normal graphite, the graphitic layers are not stacked like pages in a book, but have a more random arrangement. Linear acetylenic carbon[28] has the chemical structure[28] -(C:::C)n-. Carbon in this modification is linear with sp orbital hybridization, and is a polymer with alternating single and triple bonds. This type of carbyne is of considerable interest to nanotechnology as its Young's modulus is forty times that of the hardest known material - diamond.[30]
Occurrence



Carbon is the fourth most abundant chemical element in the universe by mass after hydrogen, helium, and oxygen. Carbon is abundant in the Sun, stars, comets, and in the atmospheres of most planets. Some meteorites contain microscopic diamonds that were formed when the solar system was still a protoplanetary disk. Microscopic diamonds may also be formed by the intense pressure and high temperature at the sites of meteorite impacts.[31]
In combination with oxygen in carbon dioxide, carbon is found in the Earth's atmosphere (approximately 810 gigatonnes of carbon) and dissolved in all water bodies (approximately 36,000 gigatonnes of carbon). Around 1,900 gigatonnes of carbon are present in the biosphere. Hydrocarbons (such as coal, petroleum, and natural gas) contain carbon as well—coal "reserves" (not "resources") amount to around 900 gigatonnes, and oil reserves around 150 gigatonnes. Proven sources of natural gas are about 175 tetrillion cubic metres (representing about 105 gigatonnes carbon), but it is estimated that there are also about 900 tetrillion cubic metres of "unconventional" gas such as shale gas, representing about 540 gigatonnes carbon.[32]
Carbon is a major component in very large masses of carbonate rock (limestone, dolomite, marble etc.).
Coal is a significant commercial source of mineral carbon; anthracite containing 92–98% carbon[33] and the largest source (4,000 Gt, or 80% of coal, gas and oil reserves) of carbon in a form suitable for use as fuel.[34]
Graphite is found in large quantities in the United States (mostly in New York and Texas), Russia, Mexico, Greenland, and India.
Natural diamonds occur in the rock kimberlite, found in ancient volcanic "necks," or "pipes". Most diamond deposits are in Africa, notably in South Africa, Namibia, Botswana, the Republic of the Congo, and Sierra Leone. There are also deposits in Arkansas, Canada, the Russian Arctic, Brazil and in Northern and Western Australia.
Diamonds are now also being recovered from the ocean floor off the Cape of Good Hope. However, though diamonds are found naturally, about 30% of all industrial diamonds used in the U.S. are now made synthetically.
Carbon-14 is formed in upper layers of the troposphere and the stratosphere, at altitudes of 9–15 km, by a reaction that is precipitated by cosmic rays. Thermal neutrons are produced that collide with the nuclei of nitrogen-14, forming carbon-14 and a proton.
Isotopes
Isotopes of carbon are atomic nuclei that contain six protons plus a number of neutrons (varying from 2 to 16). Carbon has two stable, naturally occurring isotopes.[9] The isotope carbon-12 (12C) forms 98.93% of the carbon on Earth, while carbon-13 (13C) forms the remaining 1.07%.[9] The concentration of 12C is further increased in biological materials because biochemical reactions discriminate against 13C.[35] In 1961 the International Union of Pure and Applied Chemistry (IUPAC) adopted the isotope carbon-12 as the basis for atomic weights.[36] Identification of carbon in NMR experiments is done with the isotope 13C.
Carbon-14 (14C) is a naturally occurring radioisotope which occurs in trace amounts on Earth of up to 1 part per trillion (0.0000000001%), mostly confined to the atmosphere and superficial deposits, particularly of peat and other organic materials.[37] This isotope decays by 0.158 MeV β- emission. Because of its relatively short half-life of 5730 years, 14C is virtually absent in ancient rocks, but is created in the upper atmosphere (lower stratosphere and upper troposphere) by interaction of nitrogen with cosmic rays.[38] The abundance of 14C in the atmosphere and in living organisms is almost constant, but decreases predictably in their bodies after death. This principle is used in radiocarbon dating, invented in 1949, which has been used extensively to determine the age of carbonaceous materials with ages up to about 40,000 years.[39][40]
There are 15 known isotopes of carbon and the shortest-lived of these is 8C which decays through proton emission and alpha decay and has a half-life of 1.98739x10−21 s.[41] The exotic 19C exhibits a nuclear halo, which means its radius is appreciably larger than would be expected if the nucleus were a sphere of constant density.[42]
Formation in stars
Formation of the carbon atomic nucleus requires a nearly simultaneous triple collision of alpha particles (helium nuclei) within the core of a giant or supergiant star. This happens in conditions of > 100 megakelvin temperature and helium concentration that the rapid expansion and cooling of the early universe prohibited, and therefore no significant carbon was created during the Big Bang. Instead, the interiors of stars in the horizontal branch transform three helium nuclei into carbon by means of this triple-alpha process. In order to be available for formation of life as we know it, this carbon must then later be scattered into space as dust, in supernova explosions, as part of the material which later forms second, third-generation star systems which have planets accreted from such dust. The Solar System is one such third-generation star system.
One of the fusion mechanisms powering stars is the carbon-nitrogen cycle.
Rotational transitions of various isotopic forms of carbon monoxide (e.g. 12CO, 13CO, and C18O) are detectable in the submillimeter regime, and are used in the study of newly forming stars in molecular clouds.
Carbon cycle
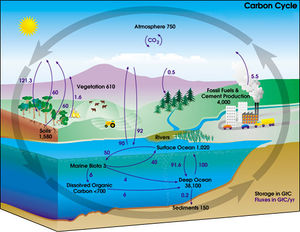
Under terrestrial conditions, conversion of one element to another is very rare. Therefore, the amount of carbon on Earth is effectively constant. Thus, processes that use carbon must obtain it somewhere and dispose of it somewhere else. The paths that carbon follows in the environment make up the carbon cycle. For example, plants draw carbon dioxide out of their environment and use it to build biomass, as in carbon respiration or the Calvin cycle, a process of carbon fixation. Some of this biomass is eaten by animals, whereas some carbon is exhaled by animals as carbon dioxide. The carbon cycle is considerably more complicated than this short loop; for example, some carbon dioxide is dissolved in the oceans; dead plant or animal matter may become petroleum or coal, which can burn with the release of carbon, should bacteria not consume it.[43]
Compounds
Organic compounds

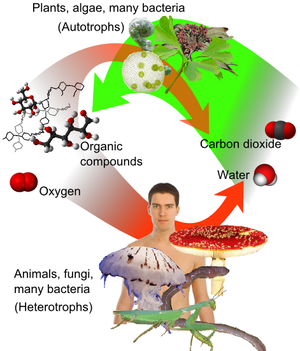
Carbon has the ability to form very long chains of interconnecting C-C bonds. This property is called catenation. Carbon-carbon bonds are strong, and stable. This property allows carbon to form an almost infinite number of compounds; in fact, there are more known carbon-containing compounds than all the compounds of the other chemical elements combined except those of hydrogen (because almost all organic compounds contain hydrogen too).
The simplest form of an organic molecule is the hydrocarbon—a large family of organic molecules that are composed of hydrogen atoms bonded to a chain of carbon atoms. Chain length, side chains and functional groups all affect the properties of organic molecules. By IUPAC's definition, all the other organic compounds are functionalized compounds of hydrocarbons.
Carbon occurs in all known organic life and is the basis of organic chemistry. When united with hydrogen, it forms various hydrocarbons which are important to industry as refrigerants, lubricants, solvents, as chemical feedstock for the manufacture of plastics and petrochemicals and as fossil fuels.
When combined with oxygen and hydrogen, carbon can form many groups of important biological compounds including sugars, lignans, chitins, alcohols, fats, and aromatic esters, carotenoids and terpenes. With nitrogen it forms alkaloids, and with the addition of sulfur also it forms antibiotics, amino acids, and rubber products. With the addition of phosphorus to these other elements, it forms DNA and RNA, the chemical-code carriers of life, and adenosine triphosphate (ATP), the most important energy-transfer molecule in all living cells.
Inorganic compounds
Commonly carbon-containing compounds which are associated with minerals or which do not contain hydrogen or fluorine, are treated separately from classical organic compounds; however the definition is not rigid (see reference articles above). Among these are the simple oxides of carbon. The most prominent oxide is carbon dioxide (CO2). This was once the principal constituent of the paleoatmosphere, but is a minor component of the Earth's atmosphere today.[44] Dissolved in water, it forms carbonic acid (H2CO3), but as most compounds with multiple single-bonded oxygens on a single carbon it is unstable.[45] Through this intermediate, though, resonance-stabilized carbonate ions are produced. Some important minerals are carbonates, notably calcite. Carbon disulfide (CS2) is similar.
The other common oxide is carbon monoxide (CO). It is formed by incomplete combustion, and is a colorless, odorless gas. The molecules each contain a triple bond and are fairly polar, resulting in a tendency to bind permanently to hemoglobin molecules, displacing oxygen, which has a lower binding affinity.[46][47] Cyanide (CN–), has a similar structure, but behaves much like a halide ion (pseudohalogen). For example it can form the nitride cyanogen molecule ((CN)2), similar to diatomic halides. Other uncommon oxides are carbon suboxide (C3O2),[48] the unstable dicarbon monoxide (C2O),[49][50] carbon trioxide (CO3),[51][52] cyclopentanepentone (C5O5),[53] cyclohexanehexone (C6O6) ,[53] and mellitic anhydride (C12O9).
With reactive metals, such as tungsten, carbon forms either carbides (C4–), or acetylides (C2−2) to form alloys with high melting points. These anions are also associated with methane and acetylene, both very weak acids. With an electronegativity of 2.5,[54] carbon prefers to form covalent bonds. A few carbides are covalent lattices, like carborundum (SiC), which resembles diamond.
Organometallic compounds
Organometallic compounds by definition contain at least one carbon-metal bond. A wide range of such compounds exist; major classes include simple alkyl-metal compounds (e.g. tetraethyllead), η2-alkene compounds (e.g. Zeise's salt, and η3-allyl compounds (e.g. allylpalladium chloride dimer; metallocenes containing cyclopentadienyl ligands (e.g. ferrocene); and transition metal carbene complexes. Many metal carbonyls exist (e.g. tetracarbonylnickel); some workers consider the carbon monoxide ligand to be purely inorganic, and not organometallic.
While carbon is understood to exclusively form four bonds, an interesting compound containing an octahedral hexacoordinated carbon atom has been reported. The cation of the compound is [(Ph3PAu)6C]2+. This phenomenon has been attributed to the aurophilicity of the gold ligands.[55]
History and etymology

The English name carbon comes from the Latin carbo for coal and charcoal,[56] and from hence also comes the French charbon, meaning charcoal. In German, Dutch and Danish, the names for carbon are Kohlenstoff, koolstof and kulstof respectively, all literally meaning coal-substance.
Carbon was discovered in prehistory and was known in the forms of soot and charcoal to the earliest human civilizations. Diamonds were known probably as early as 2500 BCE in China, while carbon in the form of charcoal was made around Roman times by the same chemistry as it is today, by heating wood in a pyramid covered with clay to exclude air.[57][58]
In 1722, René Antoine Ferchault de Réaumur demonstrated that iron was transformed into steel through the absorption of some substance, now known to be carbon.[59] In 1772, Antoine Lavoisier showed that diamonds are a form of carbon, when he burned samples of carbon and diamond then showed that neither produced any water and that both released the same amount of carbon dioxide per gram. Carl Wilhelm Scheele showed that graphite, which had been thought of as a form of lead, was instead a type of carbon.[60] In 1786, the French scientists Claude Louis Berthollet, Gaspard Monge and C. A. Vandermonde then showed that this substance was carbon.[61] In their publication they proposed the name carbone (Latin carbonum) for this element. Antoine Lavoisier listed carbon as an element in his 1789 textbook.[62]
A new allotrope of carbon, fullerene, that was discovered in 1985[63] includes nanostructured forms such as buckyballs and nanotubes.[19] Their discoverers (Curl, Kroto, and Smalley) received the Nobel Prize in Chemistry in 1996.[64] The resulting renewed interest in new forms lead to the discovery of further exotic allotropes, including glassy carbon, and the realization that "amorphous carbon" is not strictly amorphous.[26]
Production
Graphite
Commercially viable natural deposits of graphite occur in many parts of the world, but the most important sources economically are in China, India, Brazil, and North Korea.[65] Graphite deposits are of metamorphic origin, found in association with quartz, mica and feldspars in schists, gneisses and metamorphosed sandstones and limestone as lenses or veins, sometimes of a meter or more in thickness. Deposits of graphite in Borrowdale, Cumberland, England were at first of sufficient size and purity that, until the 1800s, pencils were made simply by sawing blocks of natural graphite into strips before encasing the strips in wood. Today, smaller deposits of graphite are obtained by crushing the parent rock and floating the lighter graphite out on water.
According to the USGS, world production of natural graphite in 2006 was 1.03 million tons and in 2005 was 1.04 million tons (revised), of which the following major exporters produced: China produced 720,000 tons in both 2006 and 2005, Brazil 75,600 tons in 2006 and 75,515 tons in 2005 (revised), Canada 28,000 tons in both years, and Mexico (amorphous) 12,500 tons in 2006 and 12,357 tons in 2005 (revised). In addition, there are two specialist producers: Sri Lanka produced 3,200 tons in 2006 and 3,000 tons in 2005 of lump or vein graphite, and Madagascar produced 15,000 tons in both years, a large portion of it "crucible grade" or very large flake graphite. Some other producers produce very small amounts of "crucible grade".
According to the USGS, U.S. (synthetic) graphite electrode production in 2006 was 132,000 tons valued at $495 million and in 2005 was 146,000 tons valued at $391 million, and high-modulus graphite (carbon) fiber production in 2006 was 8,160 tons valued at $172 million and in 2005 was 7,020 tons valued at $134 million.
Diamond
The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world (see figure).
Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care has to be taken in order to prevent larger diamonds from being destroyed in this process and subsequently the particles are sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of X-ray fluorescence, after which the final sorting steps are done by hand. Before the use of X-rays became commonplace, the separation was done with grease belts; diamonds have a stronger tendency to stick to grease than the other minerals in the ore.[66]
Historically diamonds were known to be found only in alluvial deposits in southern India.[67] India led the world in diamond production from the time of their discovery in approximately the 9th century BCE[68] to the mid-18th century AD, but the commercial potential of these sources had been exhausted by the late 18th century and at that time India was eclipsed by Brazil where the first non-Indian diamonds were found in 1725.[69]
Diamond production of primary deposits (kimberlites and lamproites) only started in the 1870s after the discovery of the Diamond fields in South Africa. Production has increased over time and now an accumulated total of 4.5 billion carats have been mined since that date.[70] Interestingly 20% of that amount has been mined in the last 5 years alone and during the last ten years 9 new mines have started production while 4 more are waiting to be opened soon. Most of these mines are located in Canada, Zimbabwe, Angola, and one in Russia.[70]
In the United States, diamonds have been found in Arkansas, Colorado, and Montana.[71][72] In 2004, a startling discovery of a microscopic diamond in the United States[73] led to the January 2008 bulk-sampling of kimberlite pipes in a remote part of Montana.[74]
Today, most commercially viable diamond deposits are in Russia, Botswana, Australia and the Democratic Republic of Congo.[75] In 2005, Russia produced almost one-fifth of the global diamond output, reports the British Geological Survey. Australia boasts the richest diamantiferous pipe with production reaching peak levels of 42 metric tons (41 LT; 46 ST) per year in the 1990s.[71]
There are also commercial deposits being actively mined in the Northwest Territories of Canada, Siberia (mostly in Yakutia territory, for example Mir pipe and Udachnaya pipe), Brazil, and in Northern and Western Australia. Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes.
Applications





Carbon is essential to all known living systems, and without it life as we know it could not exist (see alternative biochemistry). The major economic use of carbon other than food and wood is in the form of hydrocarbons, most notably the fossil fuel methane gas and crude oil (petroleum). Crude oil is used by the petrochemical industry to produce, amongst others, gasoline and kerosene, through a distillation process, in refineries. Cellulose is a natural, carbon-containing polymer produced by plants in the form of cotton, linen, and hemp. Cellulose is mainly used for maintaining structure in plants. Commercially valuable carbon polymers of animal origin include wool, cashmere and silk. Plastics are made from synthetic carbon polymers, often with oxygen and nitrogen atoms included at regular intervals in the main polymer chain. The raw materials for many of these synthetic substances come from crude oil.
The uses of carbon and its compounds are extremely varied. It can form alloys with iron, of which the most common is carbon steel. Graphite is combined with clays to form the 'lead' used in pencils used for writing and drawing. It is also used as a lubricant and a pigment, as a molding material in glass manufacture, in electrodes for dry batteries and in electroplating and electroforming, in brushes for electric motors and as a neutron moderator in nuclear reactors.
Charcoal is used as a drawing material in artwork, for grilling, and in many other uses including iron smelting. Wood, coal and oil are used as fuel for production of energy and space heating. Gem quality diamond is used in jewelry, and Industrial diamonds are used in drilling, cutting and polishing tools for machining metals and stone. Plastics are made from fossil hydrocarbons, and carbon fiber, made by pyrolysis of synthetic polyester fibers is used to reinforce plastics to form advanced, lightweight composite materials. Carbon fiber is made by pyrolysis of extruded and stretched filaments of polyacrylonitrile (PAN) and other organic substances. The crystallographic structure and mechanical properties of the fiber depend on the type of starting material, and on the subsequent processing. Carbon fibers made from PAN have structure resembling narrow filaments of graphite, but thermal processing may re-order the structure into a continuous rolled sheet. The result is fibers with higher specific tensile strength than steel.[76]
Carbon black is used as the black pigment in printing ink, artist's oil paint and water colours, carbon paper, automotive finishes, India ink and laser printer toner. Carbon black is also used as a filler in rubber products such as tyres and in plastic compounds. Activated charcoal is used as an absorbent and adsorbent in filter material in applications as diverse as gas masks, water purification and kitchen extractor hoods and in medicine to absorb toxins, poisons, or gases from the digestive system. Carbon is used in chemical reduction at high temperatures. Coke is used to reduce iron ore into iron. Case hardening of steel is achieved by heating finished steel components in carbon powder. Carbides of silicon, tungsten, boron and titanium, are among the hardest known materials, and are used as abrasives in cutting and grinding tools. Carbon compounds make up most of the materials used in clothing, such as natural and synthetic textiles and leather, and almost all of the interior surfaces in the built environment other than glass, stone and metal.
Diamonds
The diamond industry can be broadly separated into two basically distinct categories: one dealing with gem-grade diamonds and another for industrial-grade diamonds. While a large trade in both types of diamonds exists, the two markets act in dramatically different ways.
A large trade in gem-grade diamonds exists. Unlike precious metals such as gold or platinum, gem diamonds do not trade as a commodity: there is a substantial mark-up in the sale of diamonds, and there is not a very active market for resale of diamonds.
The market for industrial-grade diamonds operates much differently from its gem-grade counterpart. Industrial diamonds are valued mostly for their hardness and heat conductivity, making many of the gemological characteristics of diamond, including clarity and color, mostly irrelevant. This helps explain why 80% of mined diamonds (equal to about 100 million carats or 20,000 kg annually), unsuitable for use as gemstones and known as bort, are destined for industrial use.[77] In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; another 3 billion carats (600 metric tons) of synthetic diamond is produced annually for industrial use.[78] The dominant industrial use of diamond is in cutting, drilling, grinding, and polishing. Most uses of diamonds in these technologies do not require large diamonds; in fact, most diamonds that are gem-quality except for their small size, can find an industrial use. Diamonds are embedded in drill tips or saw blades, or ground into a powder for use in grinding and polishing applications.[79] Specialized applications include use in laboratories as containment for high pressure experiments (see diamond anvil cell), high-performance bearings, and limited use in specialized windows.[80][81] With the continuing advances being made in the production of synthetic diamonds, future applications are beginning to become feasible. Garnering much excitement is the possible use of diamond as a semiconductor suitable to build microchips from, or the use of diamond as a heat sink in electronics.[82]
Precautions

Pure carbon has extremely low toxicity and can be handled and even ingested safely in the form of graphite or charcoal. It is resistant to dissolution or chemical attack, even in the acidic contents of the digestive tract, for example. Consequently once it enters into the body's tissues it is likely to remain there indefinitely. Carbon black was probably one of the first pigments to be used for tattooing, and Ötzi the Iceman was found to have carbon tattoos that survived during his life and for 5200 years after his death.[83] However, inhalation of coal dust or soot (carbon black) in large quantities can be dangerous, irritating lung tissues and causing the congestive lung disease coalworker's pneumoconiosis. Similarly, diamond dust used as an abrasive can do harm if ingested or inhaled. Microparticles of carbon are produced in diesel engine exhaust fumes, and may accumulate in the lungs.[84] In these examples, the harmful effects may result from contamination of the carbon particles, with organic chemicals or heavy metals for example, rather than from the carbon itself.
Carbon may also burn vigorously and brightly in the presence of air at high temperatures, as in the Windscale fire, which was caused by sudden release of stored Wigner energy in the graphite core. Large accumulations of coal, which have remained inert for hundreds of millions of years in the absence of oxygen, may spontaneously combust when exposed to air, for example in coal mine waste tips.
The great variety of carbon compounds include such lethal poisons as tetrodotoxin, the lectin ricin from seeds of the castor oil plant Ricinus communis, cyanide (CN-) and carbon monoxide; and such essentials to life as glucose and protein.
See also
- Carbon chauvinism
- Carbon footprint
- Low-carbon economy
- Timeline of carbon nanotubes
References
- ↑ Lide, D. R., ed. (2005), CRC Handbook of Chemistry and Physics (86th ed.), Boca Raton (FL): CRC Press, ISBN 0-8493-0486-5
- ↑ 2.0 2.1 Haaland, D (1976). "Graphite-liquid-vapor triple point pressure and the density of liquid carbon". Carbon 14: 357. doi:10.1016/0008-6223(76)90010-5.
- ↑ 3.0 3.1 Savvatimskiy, A (2005). "Measurements of the melting point of graphite and the properties of liquid carbon (a review for 1963–2003)". Carbon 43: 1115. doi:10.1016/j.carbon.2004.12.027.
- ↑ "Fourier Transform Spectroscopy of the System of CP". http://bernath.uwaterloo.ca/media/36.pdf. Retrieved 2007-12-06.
- ↑ "Fourier Transform Spectroscopy of the Electronic Transition of the Jet-Cooled CCI Free Radical". http://bernath.uwaterloo.ca/media/42.pdf. Retrieved 2007-12-06.
- ↑ "Carbon: Binary compounds". http://www.webelements.com/webelements/elements/text/C/comp.html. Retrieved 2007-12-06.
- ↑ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics 81st edition, CRC press.
- ↑ 8.0 8.1 8.2 8.3 8.4 Properties of diamond, Ioffe Institute Database
- ↑ 9.0 9.1 9.2 "Carbon - Naturally occurring isotopes". WebElements Periodic Table. http://www.webelements.com/webelements/elements/text/C/isot.html. Retrieved 2008-10-09.
- ↑ "Periodic Table: Date of Discovery". Chemical Elements.com. http://www.chemicalelements.com/show/dateofdiscovery.html. Retrieved 2007-03-13.
- ↑ "Timeline of Element Discovery". http://chemistry.about.com/library/das/aa030303a.htm. Retrieved 2007-03-13.
- ↑ 12.0 12.1 "World of Carbon - Interactive Nano-visulisation in Science &Engineering Edukation (IN-VSEE)". http://invsee.asu.edu/nmodules/Carbonmod/point.html. Retrieved 2008-10-09.
- ↑ 13.0 13.1 Chemistry Operations (December 15, 2003). "Carbon". Los Alamos National Laboratory. http://periodic.lanl.gov/elements/6.html. Retrieved 2008-10-09.
- ↑ "Biological Abundance of Elements". The Internet Encyclopedia of Science. http://www.daviddarling.info/encyclopedia/E/elbio.html. Retrieved 2008-10-09.
- ↑ Greenville Whittaker, A. (1978). "The controversial carbon solid−liquid−vapour triple point". Nature 276: 695. doi:10.1038/276695a0.
- ↑ J.M. Zazula (1997). "On Graphite Transformations at High Temperature and Pressure Induced by Absorption of the LHC Beam". CERN. http://lbruno.home.cern.ch/lbruno/documents/Bibliography/LHC_Note_78.pdf. Retrieved 2009-06-06.
- ↑ C. Lee; Wei, X; Kysar, JW; Hone, J (2008). "Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene". Science 321 (5887): 385. doi:10.1126/science.1157996. PMID 18635798. http://www.sciencemag.org/cgi/content/abstract/321/5887/385. Lay summary.
- ↑ Sanderson, Bill (2008-08-25). "Toughest Stuff Known to Man : Discovery Opens Door to Space Elevator". nypost.com. http://www.nypost.com/seven/08252008/news/regionalnews/toughest_stuff__known_to_man_125993.htm. Retrieved 2008-10-09.
- ↑ 19.0 19.1 19.2 Peter Unwin. "Fullerenes(An Overview)". http://www.ch.ic.ac.uk/local/projects/unwin/Fullerenes.html. Retrieved 2007-12-08.
- ↑ 20.0 20.1 Ebbesen, TW, ed (1997). Carbon nanotubes—preparation and properties. Boca Raton, Florida: CRC Press.
- ↑ 21.0 21.1 MS Dresselhaus, G Dresselhaus, Ph Avouris, ed (2001). "Carbon nanotubes: synthesis, structures, properties and applications". Topics in Applied Physics (Berlin: Springer) 80. ISBN 3540410864.
- ↑ 22.0 22.1 Nasibulin, Albert G.; Pikhitsa, PV; Jiang, H; Brown, DP; Krasheninnikov, AV; Anisimov, AS; Queipo, P; Moisala, A et al. (2007). "A novel hybrid carbon material". Nature Nanotechnology 2 (3): 156–161. doi:10.1038/nnano.2007.37. PMID 18654245.
- ↑ Nasibulin, A; Anisimov, Anton S.; Pikhitsa, Peter V.; Jiang, Hua; Brown, David P.; Choi, Mansoo; Kauppinen, Esko I. (2007). "Investigations of NanoBud formation". Chemical Physics Letters 446: 109–114. doi:10.1016/j.cplett.2007.08.050.
- ↑ Vieira, R (2004). "Synthesis and characterisation of carbon nanofibers with macroscopic shaping formed by catalytic decomposition of C2H6/H2 over nickel catalyst". Applied Catalysis A 274: 1–8. doi:10.1016/j.apcata.2004.04.008.
- ↑ 25.0 25.1 Clifford, Frondel; Marvin, Ursula B. (1967). "Lonsdaleite, a new hexagonal polymorph of diamond". Nature 214: 587–589. doi:10.1038/214587a0.
- ↑ 26.0 26.1 26.2 Harris, PJF; Gallagher, J. G.; Hargreaves, J. S. J.; Harris, P. J. F. (2004). "Fullerene-related structure of commercial glassy carbons". Philosophical Magazine, 84, 3159–3167 116: 122. doi:10.1007/s10562-007-9125-6.
- ↑ Rode, A.V.; Hyde, S.T.; Gamaly, E.G.; Elliman, R.G.; McKenzie, D.R.; Bulcock, S. (1999). "Structural analysis of a carbon foam formed by high pulse-rate laser ablation". Applied Physics A-Materials Science & Processing 69: S755–S758. doi:10.1007/s003390051522.
- ↑ 28.0 28.1 28.2 Carbyne and Carbynoid Structures Series: Physics and Chemistry of Materials with Low-Dimensional Structures, Vol. 21 Heimann, R.B.; Evsyukov, S.E.; Kavan, L. (Eds.) 1999, 452 p., ISBN 0-7923-5323-4
- ↑ "Carbon Nanofoam is the World's First Pure Carbon Magnet". http://newton.ex.ac.uk/aip/physnews.678.html#1. Retrieved 2007-12-21.
- ↑ Lior Itzhaki; Altus, Eli; Basch, Harold; Hoz, Shmaryahu (2005). "Harder than Diamond: Determining the Cross-Sectional Area and Young's Modulus of Molecular Rods". Angew. Chem. Int. Ed. 44: 7432. doi:10.1002/ange.200502448.
- ↑ Mark (1987). Meteorite Craters. University of Arizona Press.
- ↑ "Wonderfuel: Welcome to the age of unconventional gas" by Helen Knight, New Scientist, 12 June 2010, pp. 44-7.
- ↑ R. Stefanenko (1983). Coal Mining Technology: Theory and Practice. Society for Mining Metallurgy. ISBN 0895204045.
- ↑ Kasting, James (1998). "The Carbon Cycle, Climate, and the Long-Term Effects of Fossil Fuel Burning". Consequences: the Nature and Implication of Environmental Change 4 (1). http://gcrio.org/CONSEQUENCES/vol4no1/carbcycle.html.
- ↑ Gannes, Leonard Z. (1998). "Natural Abundance Variations in Stable Isotopes and their Potential Uses in Animal Physiological Ecology". Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology 119 (3): 725–737. doi:10.1016/S1095-6433(98)01016-2.
- ↑ "Official SI Unit definitions". http://www.bipm.org/en/si/base_units/. Retrieved 2007-12-21.
- ↑ Brown, Tom (March 1, 2006). "Carbon Goes Full Circle in the Amazon". Lawrence Livermore National Laboratory. http://www.llnl.gov/str/March06/Brown.html. Retrieved 2007-11-25.
- ↑ Bowman, S. (1990). Interpreting the past: Radiocarbon dating. British Museum Press. ISBN 0-7141-2047-2.
- ↑ Libby, WF (1952). Radiocarbon dating. Chicago University Press and references therein.
- ↑ Westgren, A. (1960). "The Nobel Prize in Chemistry 1960". Nobel Foundation. http://nobelprize.org/nobel_prizes/chemistry/laureates/1960/press.html. Retrieved 2007-11-25.
- ↑ "Use query for carbon-8". http://barwinski.net/isotopes/query_select.php. Retrieved 2007-12-21.
- ↑ "Beaming Into the Dark Corners of the Nuclear Kitchen". http://www.sciencemag.org/cgi/content/full/286/5437/28?ck=nck. Retrieved 2007-12-21.
- ↑ P. Falkowski, R. J. Scholes, E. Boyle, J. Canadell, D. Canfield, J. Elser, N. Gruber, K. Hibbard, P. Högberg, S. Linder, F. T. Mackenzie, B. Moore III, T. Pedersen, Y. Rosenthal, S. Seitzinger, V. Smetacek, W. Steffen. (2000). "The Global Carbon Cycle: A Test of Our Knowledge of Earth as a System". Science 290 (5490): 291–296. doi:10.1126/science.290.5490.291. PMID 11030643.
- ↑ JS Levine, TR Augustsson and M Natarajan (1982). "The prebiological paleoatmosphere: stability and composition". Origins of Life and Evolution of Biospheres 12 (3): 245–259. doi:10.1007/BF00926894.
- ↑ T. Loerting; Tautermann, Christofer; Kroemer, Romano T.; Kohl, Ingrid; Hallbrucker, Andreas; Mayer, Erwin; Liedl, Klaus R. (2001). "On the Surprising Kinetic Stability of Carbonic Acid". Angew. Chem. Int. Ed. 39: 891–895. doi:10.1002/(SICI)1521-3773(20000303)39:5<891::AID-ANIE891>3.0.CO;2-E.
- ↑ Haldane J. (1895). "The action of carbonic oxide on man". Journal of Physiology 18 (5-6): 430–462. PMID 16992272. PMC 1514663. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1514663.
- ↑ Gorman, D; Drewry, A; Huang, YL; Sames, C (2003). "The clinical toxicology of carbon monoxide". Toxicology 187 (1): 25–38. doi:10.1016/S0300-483X(03)00005-2. PMID 12679050.
- ↑ "Compounds of carbon: carbon suboxide". http://www.webelements.com/webelements/compounds/text/C/C3O2-504643.html. Retrieved 2007-12-03.
- ↑ Bayes K. (1961). "Photolysis of Carbon Suboxide". Journal of the American Chemical Society 83: 3712–3713. doi:10.1021/ja01478a033.
- ↑ Anderson D. J.; Rosenfeld, R. N. (1991). "Photodissociation of Carbon Suboxide". Journal of Chemical Physics 94: 7852–7867. doi:10.1063/1.460121.
- ↑ Sabin, J. R. (1971). "A theoretical study of the structure and properties of carbon trioxide". Chemical Physics Letters 11 (5): 593–597. doi:10.1016/0009-2614(71)87010-0.
- ↑ Moll N. G., Clutter D. R., Thompson W. E. (1966). "Carbon Trioxide: Its Production, Infrared Spectrum, and Structure Studied in a Matrix of Solid CO2". Journal of Chemical Physics 45 (12): 4469–4481. doi:10.1063/1.1727526.
- ↑ 53.0 53.1 Alexander J. Fatiadi (1963). "Cyclic Polyhydroxy Ketones. I. Oxidation Products of Hexahydroxybenzene (Benzenehexol)". Journal of Research of the National Bureau of Standards A: Physics and Chemistry 67A (2): 153–162. http://nvl.nist.gov/pub/nistpubs/jres/067/2/V67.N02.A06.pdf.
- ↑ L. Pauling (1960). The Nature of the Chemical Bond (3rd ed.). Ithaca, NY: Cornell University Press. pp. 93.
- ↑ Franz Scherbaum, Andreas Grohmann, Brigitte Huber, Carl Krüger, Hubert Schmidbaur (1988). ""Aurophilicity" as a consequence of Relativistic Effects: The Hexakis(triphenylphosphaneaurio)methane Dication [(Ph3PAu)6C]2+". Angew. Chem. Int. Ed. Engl. 27 (11): 1544–1546. doi:10.1002/anie.198815441.
- ↑ Shorter Oxford English Dictionary, Oxford University Press
- ↑ "Chinese made first use of diamond". BBC News. 17 May 2005. http://news.bbc.co.uk/2/hi/science/nature/4555235.stm. Retrieved 2007-03-21.
- ↑ van der Krogt, Peter. "Carbonium/Carbon at Elementymology & Elements Multidict". http://www.vanderkrogt.net/elements/elem/c.html. Retrieved 2007-12-21.
- ↑ Ferchault de Réaumur, R-A (1722). L'art de convertir le fer forgé en acier, et l'art d'adoucir le fer fondu, ou de faire des ouvrages de fer fondu aussi finis que le fer forgé (English translation from 1956). Paris, Chicago.
- ↑ Senese, Fred. "Who discovered carbon?". Frostburg State University. http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/discovery-of-carbon.shtml. Retrieved 2007-11-24.
- ↑ Federico Giolitti (1914). The Cementation of Iron and Steel. McGraw-Hill Book Company, inc..
- ↑ Senese,Fred (200-09-09). "Who discovered carbon?". Frostburg State University. http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/discovery-of-carbon.shtml. Retrieved 2007-11-24.
- ↑ H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley (1985). "C60: Buckminsterfullerene". Nature 318: 162–163. doi:10.1038/318162a0.
- ↑ "The Nobel Prize in Chemistry 1996 "for their discovery of fullerenes"". http://nobelprize.org/nobel_prizes/chemistry/laureates/1996/index.html. Retrieved 2007-12-21.
- ↑ USGS Minerals Yearbook: Graphite, 2006
- ↑ G. E. Harlow (1998). The nature of diamonds. Cambridge University Press. p. 223. ISBN 0521629357.
- ↑ Catelle, W.R. (1911). The Diamond. John Lane Company. Page 159 discussion on Alluvial diamonds in India and elsewhere as well as earliest finds
- ↑ Ball, V. (1881). Diamonds, Gold and Coal of India. London, Truebner & Co.. Ball was a Geologist in British service. Chapter I, Page 1
- ↑ J. W. Hershey (1940). The Book Of Diamonds: Their Curious Lore, Properties, Tests And Synthetic Manufacture. Kessinger Pub Co. p. 28. ISBN 1417977159.
- ↑ 70.0 70.1 Janse, A. J. A. (2007). "Global Rough Diamond Production Since 1870". Gems and Gemology (GIA) XLIII (Summer 2007): 98–119.
- ↑ 71.0 71.1 Lorenz, V. (2007). "Argyle in Western Australia: The world's richest diamantiferous pipe; its past and future". Gemmologie, Zeitschrift der Deutschen Gemmologischen Gesellschaft (DGemG) 56 (1/2): 35–40.
- ↑ "Microscopic diamond found in Montana". The Montana Standard. 2004-10-17. http://www.montanastandard.com/articles/2004/10/18/featuresbusiness/hjjfijicjbhdjc.txt. Retrieved 2008-10-10.
- ↑ Cooke, Sarah (2004-10-19). "Microscopic Diamond Found in Montana". Livescience.com. http://www.livescience.com/environment/wyoming_diamond_041019.html. Retrieved 2008-09-12.
- ↑ "Delta News / Press Releases / Publications". Deltamine.com. http://www.deltamine.com/release2008-01-08.htm. Retrieved 2008-09-12.
- ↑ Marshall, Stephen; Shore, Josh (2004-10-22). "The Diamond Life". Guerrilla News Network. http://gnn.tv/videos/2/The_Diamond_Life. Retrieved 2008-10-10.
- ↑ W.J. Cantwell, J Morton (1991). "The impact resistance of composite materials - a review". Composites 22 (5): 347–62. doi:10.1016/0010-4361(91)90549-V.
- ↑ Ch. Holtzapffel (1856). Turning And Mechanical Manipulation. Charles Holtzapffel.
- ↑ "Industrial Diamonds Statistics and Information". United States Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/diamond/. Retrieved 2009-05-05.
- ↑ R. T. Coelho (1995). "The application of polycrystalline diamond (PCD) tool materials when drilling and reaming aluminum-based alloys including MMC". International journal of machine tools & manufacture 35: 761. doi:10.1016/0890-6955(95)93044-7.
- ↑ D. C. Harris (1999). Materials for infrared windows and domes: properties and performance. SPIE Press. pp. 303–334. ISBN 0819434825.
- ↑ G. S. Nusinovich (2004). Introduction to the physics of gyrotrons. JHU Press. p. 229. ISBN 0801879213.
- ↑ M. Sakamoto, J. G. Endriz, D. R. Scifres (1992). "120 W CW output power from monolithic AlGaAs (800 nm) laser diode array mounted on diamond heatsink". Electronics Letters 28 (2): 197–199. doi:10.1049/el:19920123.
- ↑ Dorfer, Leopold; Moser, M; Spindler, K; Bahr, F; Egarter-Vigl, E; Dohr, G (1998). "5200-year old acupuncture in Central Europe?". Science 282 (5387): 242–243. doi:10.1126/science.282.5387.239f. PMID 9841386.
- ↑ Donaldson, K; Stone, V; Clouter, A; Renwick, L; MacNee, W (2001). "Ultrafine particles". Occupational and Environmental Medicine 58 (3): 211–216. doi:10.1136/oem.58.3.211. PMID 11171936. PMC 1740105. http://oem.bmj.com/cgi/content/extract/58/3/211.
External links
- The Periodic Table of Videos video of Carbon at YouTube
- Carbon on Britannica
- WebElements.com – Carbon
- Extensive Carbon page at asu.edu
- Electrochemical uses of carbon
- Carbon - Super Stuff. Animation with sound and interactive 3D-models.
- BBC Radio 4 series "In Our Time", on Carbon, the basis of life, 15 June 2006
|
|||||
|
||||||||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
